Brand Name
Agrylin
Generic Name
Anagrelide
View Brand Information FDA approval date: March 14, 1997
Classification: Platelet-reducing Agent
Form: Capsule
What is Agrylin (Anagrelide)?
Anagrelide capsules are indicated for the treatment of patients with thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events. Anagrelide is a platelet reducing agent indicated for the treatment of thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events.
Approved To Treat
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
A Phase 3, Randomized, Open-label, Active-Comparator-Controlled Clinical Study to Evaluate the Safety and Efficacy of Bomedemstat (MK-3543/IMG-7289) Versus Best Available Therapy (BAT) in Participants With Essential Thrombocythemia Who Have an Inadequate Response to or Are Intolerant of Hydroxyurea
Summary: This is a study evaluating the safety and efficacy of bomedemstat (MK-3543) compared with the best available therapy (BAT) in participants with essential thrombocythemia (ET) who have an inadequate response to or are intolerant of hydroxyurea. The primary study hypothesis is that bomedemstat is superior to the best available therapy with respect to durable clinicohematologic response (DCHR).
Related Latest Advances
Brand Information
Agrylin (anagrelide hydrochloride)
1INDICATIONS AND USAGE
AGRYLIN is indicated for the treatment of patients with thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events.
2DOSAGE FORMS AND STRENGTHS
Capsules: White, opaque capsule, imprinted with "
3CONTRAINDICATIONS
None.
4CONTRAINDICATIONS
None.
5ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Cardiovascular Toxicity
- Pulmonary Hypertension
- Bleeding Risk
- Pulmonary Toxicity
5.1Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
5.2Postmarketing Experience
The following adverse reactions have been identified during post-marketing use of AGRYLIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: Prinzmetal angina, Torsades de pointes.
Respiratory, thoracic, and mediastinal disorders: Interstitial lung diseases (including allergic alveolitis, eosinophilic pneumonia, and interstitial pneumonitis) [see .
Renal and urinary disorders: Tubulointerstitial nephritis.
Hepatobiliary disorders: Clinically significant hepatotoxicity (including symptomatic ALT and AST elevations and elevations greater than three times the ULN).
Nervous system disorders: Cerebral infarction
Other adverse reactions in pediatric patients reported in spontaneous reports and literature reviews include:
Blood and lymphatic system disorders: Anemia.
Skin and subcutaneous tissue disorders: Cutaneous photosensitivity.
Investigations: Elevated leukocyte count.
6OVERDOSAGE
At higher than recommended doses, AGRYLIN has been shown to cause hypotension. There have been postmarketing case reports of intentional overdose with AGRYLIN. Reported symptoms include sinus tachycardia and vomiting. Symptoms resolved with supportive management. Platelet reduction from AGRYLIN therapy is dose-related; therefore, thrombocytopenia, which can potentially cause bleeding, is expected from overdosage.
In case of overdosage, stop AGRYLIN dosing and monitor platelet counts for thrombocytopenia and observe for possible complications such as bleeding. Consider resumption of AGRYLIN dosing once the platelet count returns to the normal range.
7DESCRIPTION
AGRYLIN (anagrelide hydrochloride) is a platelet-reducing agent. Its chemical name is 6,7-dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2(3H)-one monohydrochloride monohydrate. The molecular formula is C
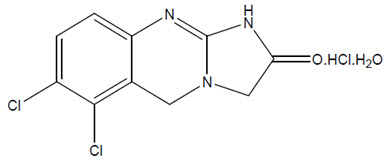
Anagrelide hydrochloride is an off-white powder. It is very slightly soluble in water and sparingly soluble in dimethyl sulfoxide and dimethylformamide.
AGRYLIN is supplied as capsules for oral administration, containing 0.5 mg of anagrelide (equivalent to 0.61 mg of anagrelide hydrochloride USP). The capsules also contain anhydrous lactose NF, crospovidone NF, lactose monohydrate NF, magnesium stearate NF, microcrystalline cellulose NF, and povidone NF as inactive ingredients. The capsule shell contains gelatin, titanium dioxide, and black iron oxide.
8HOW SUPPLIED/STORAGE AND HANDLING
AGRYLIN is available as:
0.5 mg, opaque, white capsules imprinted "
9PATIENT COUNSELING INFORMATION
- Dose: Tell the patient that their dose will be adjusted on a weekly basis until they are on a dose that lowers their platelets to an appropriate level. This will also help the patient to adjust to common side effects. Tell the patient to contact their doctor if they experience tolerability issues, so the dose or dosing frequency can be adjusted [see .
- Cardiovascular effects: Tell the patient to contact a doctor immediately if they experience chest pain, palpitations, or feel their heartbeat is irregular [see .
- Risk of Pulmonary Hypertension: Tell the patient to contact a doctor immediately if they experience shortness of breath, swelling in legs or ankles, or lips and skin turn a bluish color [see .
- Risk of bleeding: Warn the patient that concomitant aspirin (or other medicines that affect blood clotting) may increase the risk of bleeding. Tell the patient to contact a doctor immediately if they experience signs or symptoms of bleeding (e.g., vomit blood, pass bloody or black stools) or experience unexplained bruising/bruise more easily than usual [see .
- Lactation: Advise patients not to breastfeed during treatment with AGRYLIN, and for one week following the last dose [see .
- Infertility: Advise females of reproductive potential treatment with AGRYLIN may impair fertility [see .
10PRINCIPAL DISPLAY PANEL - 0.5 mg Capsule Bottle Label
063-01
Agrylin
100 CAPSULES
Rx only
0.5 mg
Takeda



